Introduction
In the world of organic synthesis, finding efficient and versatile reducing agents is crucial for a wide range of chemical transformations. Sodium Triacetoxyborohydride (STAB) is a remarkable reagent that has gained significant attention due to its effectiveness and compatibility with various functional groups. In this post, we will explore the features and applications of this powerful reducing agent, highlighting its importance in synthetic chemistry.
Understanding Sodium Triacetoxyborohydride
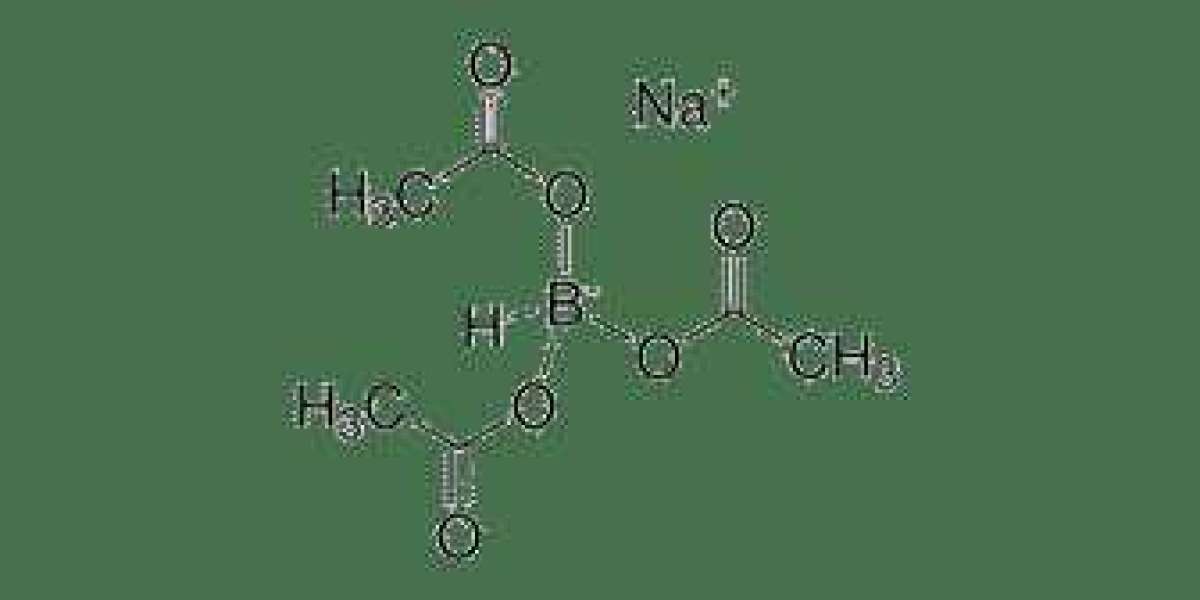
Sodium Triacetoxyborohydride, often referred to as STAB or NaBH(OAc)₃, is a versatile reducing agent used extensively in organic synthesis. STAB is known for its mild reducing properties, making it an excellent choice for transforming various functional groups without causing unwanted side reactions. This reagent is easily accessible, commercially available, and relatively inexpensive, making it a valuable tool for synthetic chemists.
How Does Sodium Triacetoxyborohydride Work?
STAB functions as a source of hydride ions (H⁻) in organic reactions. This reagent is unique because the acetate groups attached to the boron atom help activate the hydride ions, allowing them to selectively reduce a wide range of functional groups, including carbonyl compounds, imines, and nitro groups. This selectivity, combined with its mild nature, makes STAB highly versatile and applicable in various synthetic strategies.
Reduction of Carbonyl Compounds: STAB efficiently reduces aldehydes and ketones to their corresponding alcohols. This method is widely used in the synthesis of pharmaceuticals, natural products, and other complex organic molecules.
Reduction of Imines: STAB is commonly employed for the reduction of imines, producing primary amines. These reactions find application in the synthesis of amine-containing compounds, such as drugs or functional materials.
Reduction of Nitro Groups: Sodium Triacetoxyborohydride can selectively reduce nitro compounds to primary amines, a valuable transformation in the synthesis of pharmaceuticals and other nitrogen-containing organic molecules.
Reductive Amination: STAB is often utilized in reductive amination reactions, which allow for the direct conversion of carbonyl compounds to amines. This strategy has substantial synthetic potential in the construction of complex organic molecules.
Safety Considerations and Handling
While STAB is generally considered safe to handle, it is essential to follow standard safety precautions when working with any chemical reagent. Avoid direct contact with skin, eyes, and inhalation of dust or vapors. Work in a well-ventilated area and use appropriate protective equipment, such as gloves and goggles. It is also crucial to store and handle STAB in a dry and air-tight container to prevent water absorption.
Conclusion
Sodium Triacetoxyborohydride, with its mild nature and versatile reducing abilities, has become a highly valuable reagent in the field of organic synthesis. Its ability to selectively reduce various functional groups, such as carbonyl compounds, imines, and nitro groups, has opened up new avenues for the synthesis of complex organic molecules. By leveraging the power of STAB, synthetic chemists can accelerate their research and unlock innovative solutions in medicinal chemistry, materials science, and beyond. However, it is important to prioritize safety and handle this reagent responsibly, ensuring a successful and fruitful synthetic journey.